COVID-19 Search for a Cure: Roche Confirms Phase 3 of Drug Trials
Various pharmaceutical companies and research groups have worked endlessly in a bid to find a cure for the new coronavirus since Chinese health authorities first reported its existence almost four months ago.
Researchers have looked into old drugs and existing treatments in hopes it would show encouraging results in the ongoing fight against COVID-19.
Several trials have recently begun as the number of coronavirus cases hit over 420,000 worldwide.
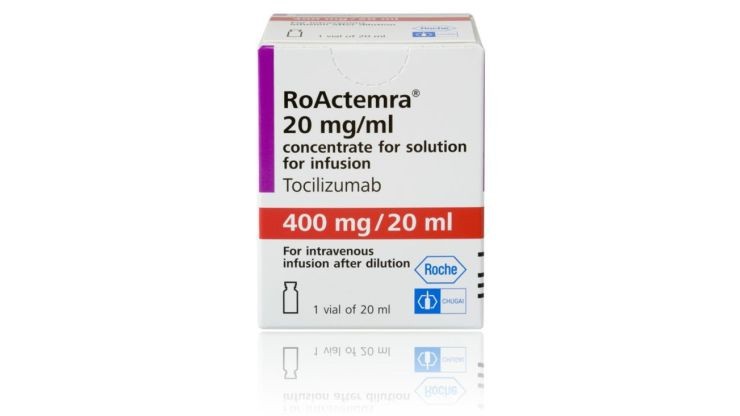
Actemra
The Swiss company, Roche, known for selling lab testing equipment, has announced the start of the third phase of its trial on a drug used to treat moderate to severe rheumatoid arthritis.
In an announcement released on March 19, the Swiss drugmaker said it was partnering with the Food and Drug Administration (FDA) and the Biomedical Advanced Research and Development Authority (BARDA) to initiate a randomized, placebo-controlled Phase III clinical trial.
The trial involves administering the drug Actemra (tocilizumab) to patients who are suffering from severe pneumonia due to COVID-19 and are receiving standard care in a hospital environment.
The drug had previously shown promising results in patients who were treated by doctors in China. According to Chinese health officials, the drug helped prevent organ failure and an overreaction of the immune system.
Roche's trial is the first global study of the drug in coronavirus-related pneumonia. Actemra is also undergoing a clinical study in Naples, Italy where 330 COVID-19 patients with pneumonia are involved. A 150-patient trial is also underway at Peking University in Beijing where researchers are administering a combination of Actemra and Fujifilm's favipiravir.
Hydroxychloroquine/Chloroquine
New York Governor Andrew Cuomo announced the start of clinical trials in New York on Tuesday. According to a news release, the state has acquired several thousand doses of hydroxychloroquine and chloroquine---drugs approved in 1949 to combat malaria---as well as large doses of Zithromax---a brand-name antibiotic drug.
The United States President Donald Trump recently promoted the anti-malaria drug during a press conference, calling it a 'game-changer.'
HYDROXYCHLOROQUINE & AZITHROMYCIN, taken together, have a real chance to be one of the biggest game changers in the history of medicine. The FDA has moved mountains - Thank You! Hopefully they will BOTH (H works better with A, International Journal of Antimicrobial Agents)..... — Donald J. Trump (@realDonaldTrump) March 21, 2020
The President recently shared a French study involving a combination of hydroxychloroquine and Zithromax on his official Twitter page. According to the results of the study, the combination of both drugs showed very encouraging results in treating patients who were positive for COVID-19.
The study was conducted after earlier reports from China documented the combo's efficacy in shortening the duration of the virus infection in patients.
Hydroxychloroquine was also tested in a clinical trial led by Didier Raoult, a prominent infectious-disease expert. The trial involved a few patients in France. The drug reportedly shortened the time patients were infectious.
Health experts warned the public to avoid self-medication. An Arizona resident, who was reported to be in his 60s, recently died after ingesting a substance that contains a form of chloroquine.
According to a news article, he took an additive used to clean fish tanks and experienced immediate effects within half an hour. His wife, who had reportedly ingested the same additive, is in critical condition.
Federal health agencies such as the Centers for Disease Control and Prevention advise residents of the United States to avoid self-medicating and seek the help of a medical professional when coronavirus-like symptoms arise.
They also remind the public to continue observing social distancing and self-isolation methods, as well as proper hygiene amid the rising number of COVID-19 cases.
Related Reads:
Subscribe to Latin Post!
Sign up for our free newsletter for the Latest coverage!
© 2026 Latin Post. All rights reserved. Do not reproduce without permission.











