FDA Cuts Waiting Period to Moderna Booster Shots to Five Months Amid COVID Cases Surge
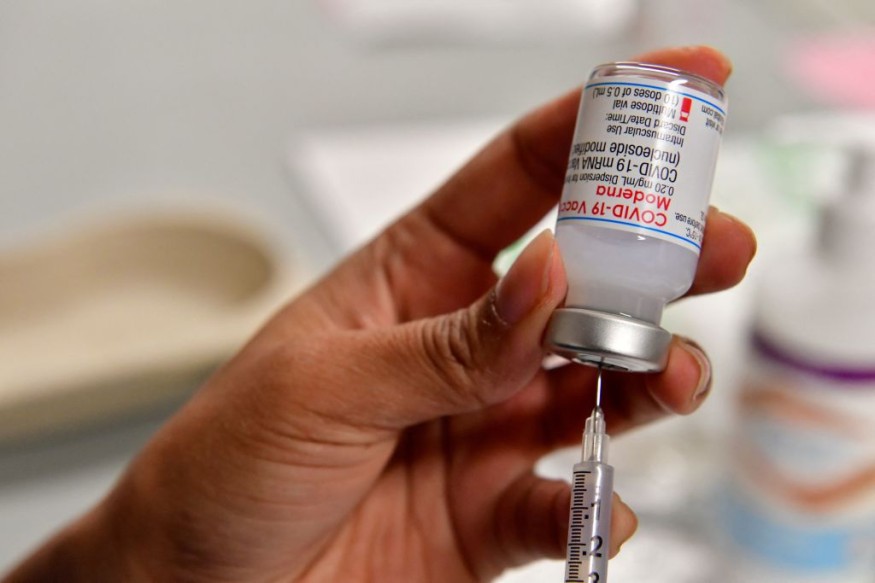
The Food and Drug Administration has shortened the waiting period to get Moderna booster shots to adults amid the COVID cases surge across the country.
The FDA has allowed everyone 12 and older who was administered with the Pfizer and BioNTech vaccine to get a booster at least five months after their second dose, which was a decrease waiting time from six months, according to a CNBC report.
The Centers for Disease Control and Prevention recommended the use of Moderna boosters for adults in October.
On Wednesday, CDC then lowered the eligibility for Pfizer boosters.
Data shows that two doses do not provide strong protection against symptomatic infection from the Omicron variant, which is a COVID variant that has grown dominant in the U.S. However, they still offer good protection against severe illness.
Peter Marks of the FDA noted that vaccination is the best defense against COVID, including the circulating variants.
The director of the agency's research department added that shortening the length of time for boosters may help reduce the decrease of immunity over time, according to a Reuters report.
Boosters were shown to be up to 75 percent effective at preventing such infection, according to the U.K. Health Security Agency's published report.
Moderna Booster
Moderna CEO Stephane Bancel noted that a fourth dose may be needed at some point after finding out that boosters are likely to decline its protection over time.
The U.K. Health Security agency found that booster protection starts to decline about four weeks, with boosters being 55 percent to 70 percent effective at preventing infection at weeks five to nine.
It then decreases to 40 to 50 percent effective 10 weeks after receiving the shot.
Pfizer CEO Albert Bourla echoed the Moderna CEO's sentiments. Bourla said people may need a fourth shot sooner than expected.
Meanwhile, CDC supported the shortened waiting period to get a booster.
Omicron Variant in The U.S.
The United States is currently facing an unprecedented wave of COVID infections, with a seven-day average of over 600,000 new cases every day. This is a 72 percent increase from the previous week and a pandemic record.
Meanwhile, California is extending its indoor mask mandate to mid-February amid the rise of COVID cases that could overwhelm health care facilities.
State models forecast hospitalizations could be around 20,000 by early next month.
The World Health Organization Director-General, Tedros Adhanom Ghebreyesus, warned that even though the Omicron variant appears to cause less severe symptoms, it should not be categorized as "mild," according to an Aljazeera report. Ghebreyesus also repeated his call for greater global equity in the distribution of and access to COVID vaccines.
Ghebreyesus noted that 109 countries will miss WHO's target for 70 percent of the world's population to be fully vaccinated by July with the current vaccination rollout.
This article is owned by Latin Post.
Written by: Mary Webber
WATCH: FDA advisers endorse Moderna's COVID-19 vaccine booster shots - from CBS News
Subscribe to Latin Post!
Sign up for our free newsletter for the Latest coverage!
© 2025 Latin Post. All rights reserved. Do not reproduce without permission.














